Abstract
Background
Growing evidence suggests that geriatric assessment (GA) based on Activity of Daily Living (ADL), Instrumental ADL (IADL), and comorbidity index can predict survival in elderly patients with diffuse large B-cell lymphoma (DLBCL). However, studies regarding GA in elderly DLBCL patients are scarce in Asia, and the impact of relative doxorubicin dose intensity (RDDI) based on GA has never been evaluated in Asian elderly DLBCL patients. Therefore, this prospective cohort study was conducted to develop a geriatric risk model integrating simplified GA to predict event-free survival (EFS) and to evaluate the impact of RDDI on EFS based on developed risk model.
Methods
Patients with untreated de novo DLBCL were enrolled onto the 2 prospective cohorts (GERIAD1 [NCT02555267] between Sep 2015 and Jul 2017 and GERIAD2 [NCT03211702] between Aug 2017 and Apr 2018) from 13 Korean institutions if they were aged ≥65 years and intended to receive rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) therapy. After enrolment, a simplified GA was performed using the Korean versions of the Katz ADL and the Lawton IADL and the Charlson Comorbidity Index (CCI). Dose and interval of R-CHOP therapy was left to the treating physician's discretion and did not have to be based on GA results. The primary end-point was EFS (events defined as death from any cause, relapse from complete response, progression during or after treatment, and unplanned changes of therapy during R-CHOP). Secondary end-points included overall survival (OS), progression-free survival (PFS), and RDDI.
Results
249 patients were enrolled. The median age was 75 years (range, 65-88) and 34 (14%) patients were >80 years. Approximately half of patients were male (N=124) and had elevated LDH level (N=147) and advanced stage (N=140). Based on the age-adjusted International Prognostic Index (aaIPI), 119 (48%) patients were classified as high-intermediate or high risk groups. 70 (28%) patients had an ADL score <5, 84 (34%) an IADL score <7, and 45 (18%) a CCI ≥2.
With a median follow-up of 41.7 months (IQR 34.6-49.2), the 2-year EFS, PFS, and OS was 53.5%, 55.9% and 63.9%, respectively. In univariable analysis, EFS was significantly worse in patients aged 71-80 (HR 1.67, p=0.018) and in patients with >80 (HR 2.62, p=0.001), compared to patients with ≤70. Patients with ADL <5 (HR 3.38, p<0.001), IADL <7 (HR 3.84, p<0.001), and CCI ≥2 (HR 1.95, p=0.001) had also worse EFS than those with ADL 5-7, IADL 7-10, and CCI <2. In addition to these variables, individual aaIPI factors, total aaIPI score, presence of B symptoms, hemoglobin level, and serum albumin level were significantly associated with EFS.
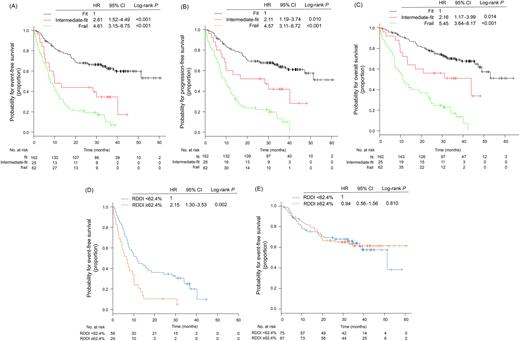
In the multivariable Cox analysis for EFS, age, ADL, IADL, CCI, and aaIPI were independently associated with EFS (for age 71 to 80 years, HR 1.51 [95%CI 0.94-2,43]; age >80 years, HR 2.21 [95%CI 1.20-4.07]: for ADL <5, HR 1.82 [95%CI 1.02-3.28]: for IADL <7, HR 1.79 [95%CI 0.99-3.27]: for CCI ≥2, HR 1.47 [95%CI 0.97-2.23]). To build a geriatric risk model, the Cox model was re-fit with only age (for age 71 to 80 years, HR 1.55, p=0.068; age >80 years, HR 2.44, p=0.003), ADL (for ADL <5, HR 1.89, p=0.025), IADL (for IADL <7, HR 2.36, p=0.003), and CCI (for CCI ≥2, HR 1.59, p=0.027), and we then determined risk score by assigning different weights to each risk factor based on their HR from final Cox model. Based on these results, we stratified patients into 3 groups according to risk score: fit (≤33rd percentile, n=162, 65%), intermediate-fit (>33rd percentile and ≤66th percentile, n=25, 10%), and frail (>66th percentile, n= 62, 25%), with 2-year EFS of 67.7%, 44.0%, and 21.0%, respectively (Fig A). This model was also significantly associated with PFS and OS (Fig B and C).
The median RDDI was 62% (range, 27-101%), which was significantly higher in fit patients (66%) than intermediate-fit or frail patients (60%). In 87 intermediate-fit or frail patients, higher RDDI (≥62.4%) was associated with worse EFS (2-year, 10.3%, vs. 36.2%; p=0.002; Fig D) compared with lower RDDI (<62.4%). However, there was no significant difference in EFS between 162 fit patients with RDDI ≥62.4% or < 62.4% (2-year, 66.4%, vs. 69.5%; p=0.810; Fig E).
Conclusion
We propose a geriatric risk model integrating simplified GA and age, which successfully predict the survival outcomes in Asian elderly patients with DLBCL. The model may serve as a tool to guide risk-adapted treatment approaches.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.